
Gallium is a chemical element; it has symbolGa and atomic number 31. Discovered by the French chemistPaul-Émile Lecoq de Boisbaudran in 1875,[10] gallium is in group 13 of the periodic table and is similar to the other metals of the group (aluminium, indium, and thallium).-1
Gallium is a soft, silvery metal used primarily in electronic circuits, semiconductors and light-emitting diodes (LEDs). It is also useful in high-temperature thermometers, barometers, pharmaceuticals and nuclear medicine tests. The element has no known biological value.
In nature, gallium is never found as a free element and cannot be found in a substantial amount in any minerals. Rather, it exists in trace amounts in various compounds, including zinc ores and bauxite. -2
Gallium has no known biological role. It is non-toxic. -3
Materials to Melt Gallium in Your Hand
All you need to melt gallium in your hand is a reasonably pure chunk of the element.
- Gallium
- Plastic gloves (optional)
Gallium does not exist free in nature. It results from the purification of other metal ores. You won’t easily find it in a store, there are links available below for purchase suggestions in various quantities.
While skin contact with gallium is safe, there are two reasons why you might want to wear gloves anyway. First, gallium wets skin. Contact gives skin a grayish cast that isn’t necessarily easily washed away. Second, gallium damages metals. If you normally wear a ring, either remove it before holding gallium or else wear gloves to protect it from discoloration.

How to Melt Gallium in Your Hand
Place a chunk of gallium in the palm of your hand and let body heat do all the work. Gallium melts almost immediately in a very warm room, but it may take 3-5 minutes to melt a coin-sized piece of metal in a cool room.
Feel free to tilt your hand and watch the metal flow. When you are finished, cup your hand and pour the gallium into a container. If the container is also warm, watch for metal crystal formation as the element cools. Gallium displays an orthorhombic crystal structure. Its crystal facets are not sharp like you see in other metals, but have a sort of soft, melted appearance. Over time, the shiny metal surface dulls as an oxide layer builds.
Repeat the project as many times as you like. Gallium is a pure element, so it’s not going anywhere.
There are many other gallium science projects found at https://sciencenotes.org/melt-gallium-in-your-hand-gallium-science-projects/
Where to buy Gallium?

10g gallium, purity grade 4N/99.99%, melting bead for element collectors. Melting point 29.8°C. Delivery from Germany. £12.99
Magnametals Gallium 99.99% (4N) Pure 20g – £25
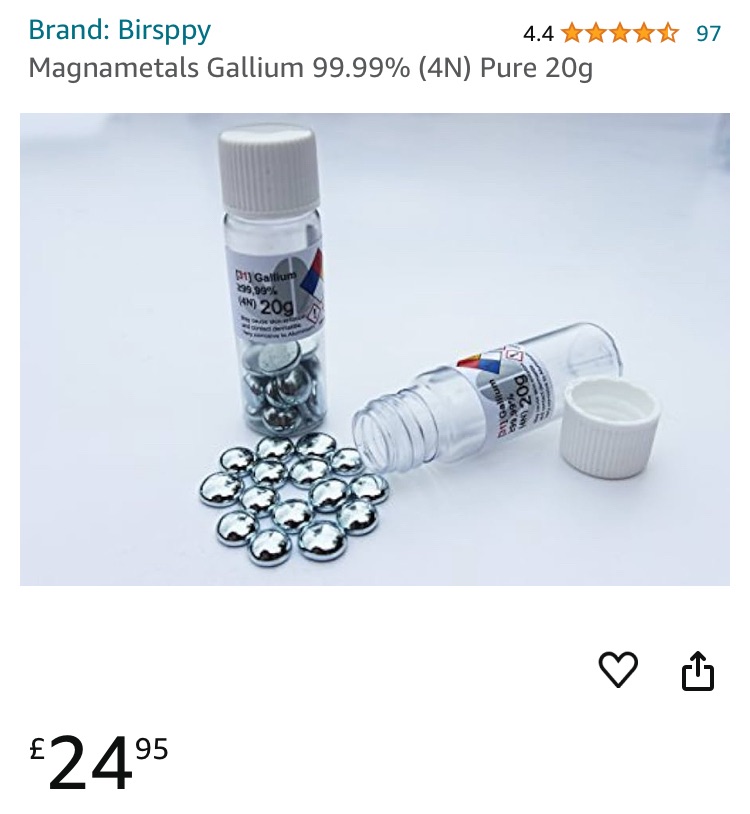
It is sold in a screw cap plastic bottle so you can look at the pure gallium beads. For your convenience you can easilyamount of gallium you want to work with, rather than having to melt a large solid lump of metal. We include a polyethylene bottle and cap for melting the gallium and storing melted gallium afterwards. We advise you do not handle with your hands directly. Firstly, as it covers your hands in a fine grey covering that is difficult to remove. Secondly it will contaminate the pure gallium. Use the Nitrile gloves that we supply or a clean plastic spatula or spoon. You will receive a 4N gallium Certificate of analysis (COA) and a Material Safety Data Sheet (MSDS) with your gallium.
Really Recommended Resources
As part of our exciting #IYPT2019 celebrations, we’re partnering with the brilliant Compound Interest to bring you these fantastic new element infographics, in Periodic Table order, from H to Og.
Download the PDFs to make your own wall displays or education resources.
Sources taken from:
1- https://en.m.wikipedia.org/wiki/Gallium
2- https://www.livescience.com/29476-gallium.html
3- https://www.rsc.org/periodic-table/element/31/gallium
4- https://sciencenotes.org/melt-gallium-in-your-hand-gallium-science-projects/
5- https://www.bealsscience.com/post/2017/12/06/gallium-amazing-metal-melts-in-your-hand




Leave a comment